Systemic lupus erythematosus (SLE) carries a substantial hereditary component, with estimates showing up to 66 percent of risk derived from genetic predisposition. Families and patients often face uncertainty about why lupus appears in siblings or generations, and understanding lupus hereditary factors to clarify those concerns. This article delivers clear definitions, mechanisms, and real-world examples of genetic risk, gene roles, environmental interplay, testing options, research advances, and demographic disparities. It also highlights how crwf.com’s clinical studies deepen family support and pave the way for personalized therapies.
We begin by examining how inherited variants raise lupus risk. Then we explore the key genes implicated and their functions. Next, we discuss environmental and hormonal interactions that trigger disease expression. We review genetic testing and counseling options for families, followed by the latest clinical trials and precision medicine applications. Finally, we cover ethnic and gender differences in lupus genetics and share resources for families navigating hereditary lupus.
What Is the Genetic Predisposition to Lupus and How Does It Affect Risk?
Genetic predisposition to lupus means inheriting specific gene variants that increase the likelihood of developing SLE by altering immune regulation and inflammatory pathways. This predisposition explains why close relatives of lupus patients carry elevated risk compared to the general population. For example, first-degree relatives have a 20 times higher chance of developing SLE, illustrating how inherited factors shape disease probability.
Genetic susceptibility involves multiple loci across the genome, each contributing modestly to overall risk. Together, these variants disrupt tolerance to self-antigens, promoting autoimmunity. Family history serves as a practical indicator of inherited lupus risk, guiding early monitoring and supportive care. Acknowledging this genetic foundation empowers families to seek appropriate counseling and participate in preventive clinical research.
Genetic Predisposition in Systemic Lupus Erythematosus – en
Research indicates that genetic factors significantly contribute to the risk of developing systemic lupus erythematosus (SLE), with studies showing a substantial heritability component. These genetic predispositions influence immune regulation and inflammatory pathways, increasing the likelihood of SLE in individuals with specific gene variants.
Tsao, B. P., et al., Genes and Immunity (2008)
What Does Genetic Susceptibility Mean in Systemic Lupus Erythematosus?
Genetic susceptibility in SLE refers to the presence of inherited DNA variations that affect immune cell function and promote self-reactivity. These variations can alter antigen presentation, cytokine signaling, or complement activation, which in turn compromise the body’s ability to distinguish self from foreign. For instance, certain HLA alleles decrease clearance of immune complexes, contributing to chronic inflammation and tissue damage.
Susceptibility is polygenic: hundreds of common variants each add a small risk increment, and rare mutations such as those in TLR7 may drive severe disease forms. Knowing one’s genetic profile helps patients and families recognize why symptoms may appear and guides participation in targeted studies. Understanding susceptibility sets the stage for exploring how specific inheritance patterns shape lupus within families.
How Do Inheritance Patterns Influence Lupus Development in Families?
Inheritance patterns in lupus are complex and non-Mendelian, often described as polygenic or multifactorial. No single gene “causes” SLE; instead, dozens of loci jointly raise risk. First-degree relatives of lupus patients have about a 5–10 percent lifetime risk, compared to 0.1 percent in the general population. This familial aggregation reflects shared genetics and environmental exposures.
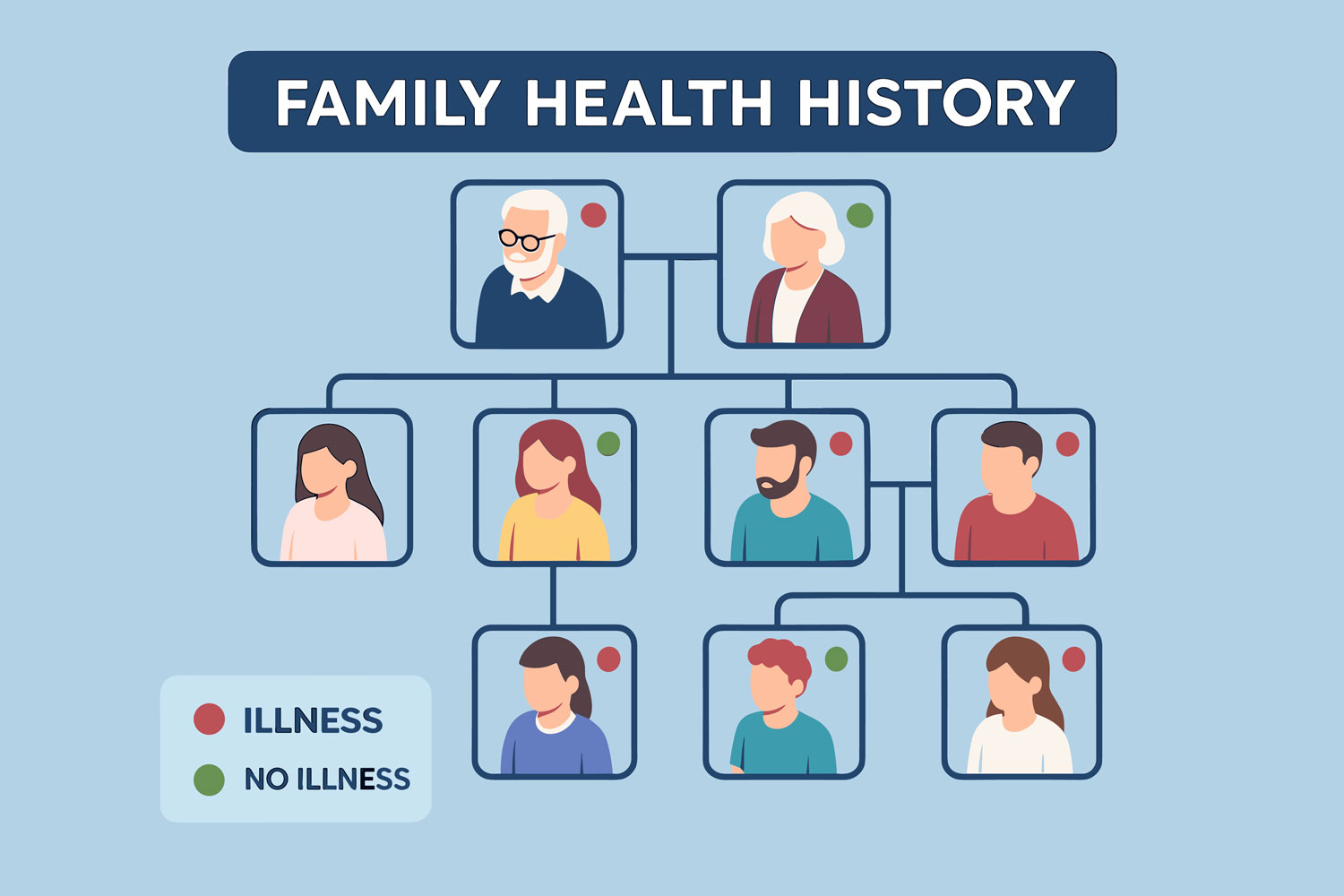
Studies of pedigrees reveal that both parents can pass on variants that combine in offspring to exceed a threshold for disease expression. Siblings share roughly 50 percent of these risk variants, explaining why sibling concordance is higher than parent-child concordance. Recognizing these inheritance dynamics helps families understand why lupus may cluster in a household and supports early screening for at-risk relatives.
What Do Twin Studies Reveal About Lupus Heritability?
Twin studies offer clear evidence of lupus heritability by comparing concordance rates between monozygotic (identical) and dizygotic (fraternal) twins. Identical twins share 100 percent of their genes, while fraternal twins share about 50 percent. Higher concordance in monozygotic pairs confirms a strong genetic component.
Below is a concise comparison of twin concordance:
| Twin Type | Concordance Rate | Implication for Heritability |
|---|---|---|
| Monozygotic | 24–69 percent | Indicates high genetic influence |
| Dizygotic | 2–5 percent | Reflects baseline shared genetics |
These rates demonstrate that genetics account for a major portion of lupus risk, while the gap between twin types highlights the role of non-genetic factors. Understanding heritability via twin studies prepares readers to explore which genes specifically contribute to SLE.
Twin Studies and Lupus Heritability – en
Twin studies provide strong evidence for the heritability of lupus, with higher concordance rates in monozygotic (identical) twins compared to dizygotic (fraternal) twins. This difference highlights the significant genetic influence on lupus development, while also acknowledging the role of non-genetic factors.
Deapen, D., et al., Arthritis & Rheumatism (1992)
Which Genes Are Linked to Lupus and What Roles Do They Play?
Multiple genes contribute to lupus susceptibility by regulating immune responses and inflammatory pathways. Variants in these genes can impair pathogen sensing, antigen clearance, and immune tolerance, setting the stage for SLE. Recognizing the key genes involved provides insight into disease mechanisms, potential biomarkers, and therapeutic targets.
How Do MHC and HLA Genes Affect Lupus Susceptibility?
MHC genes, especially HLA-DR2 and HLA-DR3 alleles on chromosome 6, shape antigen presentation to T cells. Variants in these genes alter the set of peptides displayed on cell surfaces, leading to presentation of self-antigens and subsequent autoimmune activation. People carrying high-risk HLA alleles have up to a fourfold increased lupus risk.
This mechanism directly influences the strength and specificity of T-cell responses. HLA genotype testing can stratify patients in clinical trials, ensuring cohorts share similar antigen-presentation profiles. Recognizing HLA’s role sets the foundation for personalized intervention strategies based on individual genotype.
What Is the Role of the TLR7 Gene in Lupus Pathogenesis?
TLR7 encodes a toll-like receptor that senses single-stranded RNA and triggers innate immune activation. Gain-of-function mutations in TLR7 increase signaling through type I interferon pathways, a hallmark of SLE. Recent discoveries show that certain TLR7 variants directly drive severe, early-onset lupus by amplifying inflammation in B cells and dendritic cells.
By stimulating interferon production, TLR7 variants promote autoantibody generation and tissue damage. These insights offer a clear target for novel therapies, as inhibiting TLR7 signaling can reduce disease activity. Clinical studies at crwf.com leverage TLR7 genotyping to test interferon-blockade agents in genetically defined patient groups.
How Do Complement Genes (C1q, C2, C4) Contribute to Lupus Risk?
Complement genes encode proteins that clear immune complexes and apoptotic debris. Deficiencies or polymorphisms in C1q, C2, and C4 impair this clearance, resulting in persistent inflammation and autoantibody deposition in tissues.
Key complement contributions include:
- C1q deficiency leads to defective clearance of apoptotic cells, promoting antigen presentation.
- C2 variants reduce overall complement activation, weakening immune complex removal.
- C4 copy-number variations correlate with altered complement levels and SLE severity.
These complement defects illustrate how impaired debris clearance facilitates autoimmunity and emphasize the benefit of complement-targeted therapies under investigation.
What Other Genetic Markers Are Associated with Lupus?
Beyond MHC, TLR7, and complement, several transcription and signaling genes influence lupus risk. Notable markers include:
- IRF5 – Regulates interferon response genes, driving chronic inflammation.
- STAT4 – Transmits cytokine signals essential for Th1 cell differentiation.
- PTPN22 – Modulates T-cell receptor signaling, affecting self-tolerance.
Each marker adds a piece to the polygenic risk puzzle and offers potential for biomarker panels that improve early diagnosis and personalized treatment selection.
How Do Environmental and Hormonal Factors Interact with Lupus Genetics?
Environmental and hormonal influences modulate gene expression and immune activation in individuals carrying lupus-associated variants. These interactions often involve epigenetic modifications, such as DNA methylation changes, that alter gene transcription without modifying the DNA sequence itself.
For example, ultraviolet radiation can induce epigenetic changes in skin immune cells, triggering flares in genetically predisposed patients. Likewise, estrogen enhances B-cell survival and antibody production through receptor-mediated gene regulation. Understanding these interactions allows families to adopt targeted avoidance strategies and informs the design of clinical studies investigating gene-environment mechanisms.
What Environmental Triggers Can Activate Lupus in Genetically Predisposed Individuals?
Environmental triggers expose underlying genetic risk by inducing stress responses or molecular damage. Common lupus triggers include:
- Ultraviolet (UV) radiation from sunlight
- Viral infections such as Epstein-Barr virus
- Tobacco smoke and silica dust
- Certain medications that alter immune balance
Recognizing and minimizing these triggers helps at-risk individuals reduce flare frequency and informs trial protocols that evaluate gene-environment interventions.
How Does Estrogen Influence Lupus Severity and Genetic Risk?
Estrogen regulates gene expression via estrogen receptors on immune cells, enhancing B-cell activation and autoantibody production. The X chromosome also carries numerous immune-related genes, and estrogen’s effects further skew immune tolerance. Women, who have two X chromosomes and higher estrogen levels, are nine to ten times more likely to develop lupus, highlighting hormone-gene synergy in disease pathogenesis.
This hormonal influence explains gender differences in incidence and suggests that modulating estrogen signaling could complement genetic risk mitigation strategies.
What Are Gene-Environment Interactions in Lupus Development?
Gene-environment interactions occur when external factors modify the expression or function of lupus-related genes. Epigenetic mechanisms such as DNA methylation, histone modifications, and microRNA regulation alter how genes like IRF5 or PTPN22 respond to stimuli. For instance, smoking-induced epigenetic changes can upregulate proinflammatory genes in predisposed individuals, triggering flares.
Clinical studies now measure epigenetic markers alongside genetic profiles to identify high-risk combinations and test interventions that restore normal gene regulation.
What Are the Options for Genetic Testing and Counseling in Lupus Families?
Is Genetic Testing Available for Lupus Risk Assessment?
While no routine clinical test predicts lupus with certainty, research laboratories offer panels that screen for variants in genes such as HLA-DR, TLR7, IRF5, and complement components. These tests inform patient stratification in clinical trials and guide personalized monitoring plans. Testing requires consultation with genetics professionals to understand limitations and implications.

How Can Families Understand Their Lupus Risk Through Genetic Counseling?
Genetic counselors explain inheritance patterns, interpret polygenic risk scores, and help relatives weigh the benefits and uncertainties of testing. Counseling sessions cover:
- The difference between predisposition and disease expression
- Risk estimates for first-degree relatives
- Potential lifestyle adjustments to reduce environmental triggers
This personalized guidance supports informed decision-making and links families to crwf.com’s genetics-focused clinical studies.
What Should Families Expect Regarding Hereditary Lupus Risk?
Families should recognize that genetic predisposition elevates risk but does not guarantee disease. Counseling clarifies:
- Predisposition reflects increased probability, not a diagnosis
- Environmental and hormonal factors influence whether predisposition leads to SLE
- Early monitoring and research trial participation can improve outcomes
Understanding these nuances prepares families to engage proactively in research and preventive care.
How Is Genetic Research Advancing Lupus Treatment and Clinical Trials?
Genetic research drives precision medicine approaches that tailor therapies based on individual genetic profiles. By stratifying patients according to key variants—such as TLR7 or complement gene status—clinical trials at crwf.com test targeted agents more efficiently and identify responders with higher precision. This strategy accelerates the development of effective, low-toxicity treatments.
What Current Clinical Studies Explore Lupus Genetics?
Several ongoing trials investigate interventions matched to genetic subtypes:
- Interferon-blockade in TLR7-variant carriers – Tests monoclonal antibodies that neutralize type I interferon.
- Complement replacement in C1q-deficient patients – Assesses recombinant C1q to restore debris clearance.
- Epigenetic modulators – Trials drugs that reverse DNA methylation changes in immune cells.
These studies demonstrate how genetic insights guide novel therapy development and improve clinical trial success rates.
How Does Precision Medicine Use Genetic Profiles to Tailor Lupus Treatments?
Precision medicine integrates genetic data with clinical features to match patients to therapies most likely to help. For example:
- TLR7 inhibitors benefit those with gain-of-function mutations.
- B-cell targeted therapies suit patients with high IRF5 activity.
- Complement modulators address complement gene deficiencies.
This approach enhances efficacy and reduces unnecessary exposure to broad immunosuppression.
What Is the Future of Gene Therapy and Genetic-Based Lupus Treatments?
Emerging gene-editing techniques, such as CRISPR/Cas9, offer long-term correction of rare lupus-driving mutations. Early research aims to repair gain-of-function TLR7 variants or restore complement gene expression in patient-derived cells. Although still experimental, these innovations promise durable cures by addressing root genetic causes.
Why Are Ethnic and Gender Differences Important in Lupus Genetics?
Ethnic and gender disparities in lupus prevalence and severity reflect underlying genetic variation and hormonal influences. Recognizing these differences ensures equitable research participation and development of treatments effective across diverse populations. Tailored trial recruitment and subgroup analyses identify unique genetic markers and improve outcomes for all demographic groups.
How Do Genetic Factors Explain Lupus Prevalence in Different Ethnic Groups?
Individuals of African, Asian, Hispanic/Latino, Native American, and Pacific Islander descent have two to three times higher lupus rates compared to European ancestry, partly due to population-specific risk alleles. For example, certain IRF5 haplotypes are more common in African-American patients. Identifying these alleles guides inclusive trial design and the creation of ancestry-informed diagnostic tools.
Why Are Women More Genetically Susceptible to Lupus Than Men?
Women’s two X chromosomes harbor numerous immune-regulatory genes, and incomplete X-inactivation can lead to gene dosage effects that predispose to autoimmunity. Estrogen further amplifies B-cell activity by upregulating autoantibody production genes. These combined genetic and hormonal influences explain the nine-to-one female predominance and underscore the need for gender-specific research strategies.
What Research Is Being Done on Genetic Disparities in Lupus?
Researchers at crwf.com and partner institutions conduct genome-wide association studies in underrepresented populations, aiming to discover novel variants and validate known markers across ancestries. Trials now include diverse cohorts to evaluate how genetic differences affect treatment response, ensuring that emerging therapies benefit all ethnic groups equally.
What Resources and Support Are Available for Families Affected by Hereditary Lupus?
Families navigating hereditary lupus can access genetic counseling services, enroll in clinical research, and explore educational materials designed for non-specialists. These resources help relatives understand risk, connect with specialized care teams, and join trials that shape the future of SLE treatment.
Where Can Families Find Genetic Counseling and Lupus Risk Assessment?
Genetic counseling centers affiliated with academic medical centers and specialized clinics offer SLE risk assessment. Counselors work with rheumatologists and immunologists to interpret test results and guide preventive strategies. crwf.com’s referral network connects families to certified genetic counselors experienced in autoimmune disorders.
How Can Patients Participate in Lupus Genetic Research and Clinical Trials?
Patients and relatives can enroll in observational studies and interventional trials at crwf.com’s research sites. Participation opportunities are listed on the website’s clinical studies section and include genotype-guided protocols. Joining research not only advances scientific knowledge but may provide access to cutting-edge therapies.
What Educational Materials Help Families Understand Lupus Genetics?
A variety of resources support family education:
- Illustrated guides explaining genetic concepts
- Videos of researchers discussing gene roles in SLE
- Glossaries of medical terms tailored to patients
- Patient stories highlighting hereditary patterns
These materials empower families with clear information and foster engagement in supportive networks.
SLE’s hereditary factors underscore the importance of genetic insight for families and patients. Identifying predisposition through family history, gene profiling, and counseling enables early monitoring and personalized preventive strategies. Environmental and hormonal triggers interact with inherited variants to shape disease expression, guiding lifestyle adjustments and research protocols. Advances in precision medicine and targeted clinical trials promise more effective treatments tailored to individual genetic profiles, while attention to ethnic and gender differences ensures equitable benefits. Ongoing support from counseling services, educational resources, and crwf.com’s clinical studies empowers families to navigate hereditary lupus with confidence and hope for future breakthroughs.
If you have Lupus and are looking for a clinical trial, contact us today!


